| (2023.09) Photocatalytic H2O2 production from water and air using porous organic polymers | |||||
| 작성자 | 관리자 | 작성일 | 2024-06-10 | 조회수 | 53 |
|---|---|---|---|---|---|
|
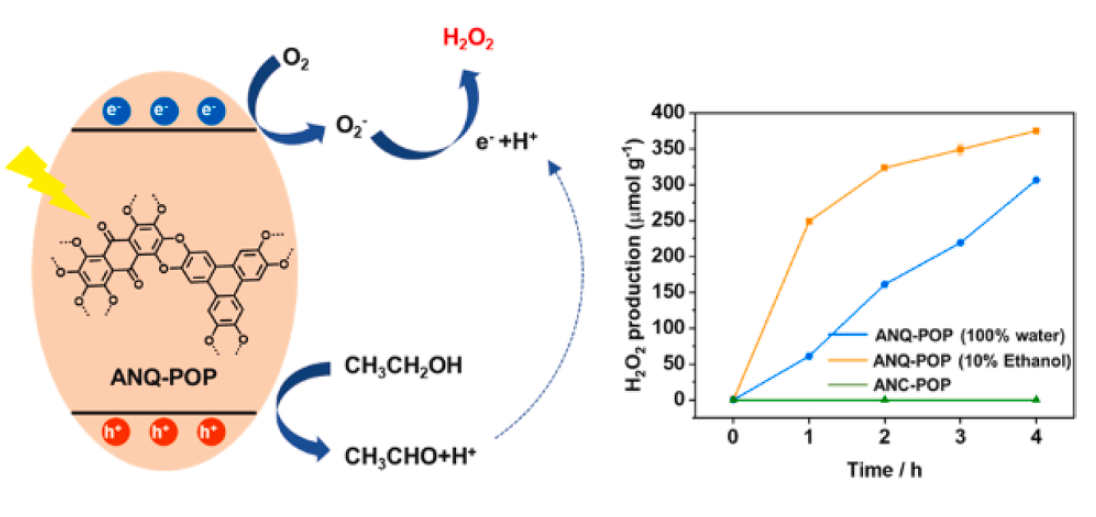
· 논문명 : Photocatalytic H2O2 production from water and air using porous organic polymers · 저 자 : Bishal Boro, Nayeong Kim, Jae-Seung Kim, Ratul Paul, Yogendra Nailwal, Yuri Choi, Dong-Hwa Seo, John Mondal*, Jungki Ryu* · 게재지 : Journal of Colloid And Interface Science (2023, 652, 1784-1792) · 초록 Producing hydrogen peroxide (H2O2) from H2O and O2 under visible light irradiation is a promising solar-to-chemical energy conversion technology. Hydrogen peroxide has versatile applications as a green oxidant and liquid energy carrier but has been produced through energy-intensive and complex anthraquinone processes. Herein, we report the rational design of efficient and stable porous organic polymer(POP) containing redox centers, anthraquinone photocatalyst (ANQ-POP) for solar H2O2 production. ANQ-POP is readily synthesized with stable dioxin-linkages via efficient one-pot, transition-metal-free nucleophilic aromatic substitution reactions between 1,2,3,4,5,6,7,8-octafluoro-9,10-anthraquinone (OFANQ) and 2,3,6,7,10,11-hexahydroxytriphenylene (HHTP). Exhibiting a fibrillar morphology,ANQ-POP boasts a high surface area of 380 m2·g-1 and demonstrates thermal stability. With 10 % ethanol, ANQ-POP yields an H2O2 production rate of 320 μmol g-1 under visible light irradiation. Moreover, ANQ-POP alone can efficiently produce H2O2 without any photosensitizers and cocatalysts. Density functional theory calculations reveal that the quinone groups of the anthraquinone moieties can serve as redox centers for H2O2 production under light irradiation. Furthermore, unlike most conventional photocatalysts, it can produce H2O2 using only water and air by catalyzing both oxygen reduction and evolution reactions under light irradiation. Our findings provide an efficient, eco-friendly pathway for photocatalytic production of H2O2 under mild reaction conditions using a dioxinderived POP-based photocatalyst.
|
|||||