| (2023.08) Role of Physicochemical Features toward Bifunctional Redox Activity of Transition-Metal Molybdates | |||||
| 작성자 | 관리자 | 작성일 | 2024-04-09 | 조회수 | 52 |
|---|---|---|---|---|---|
|
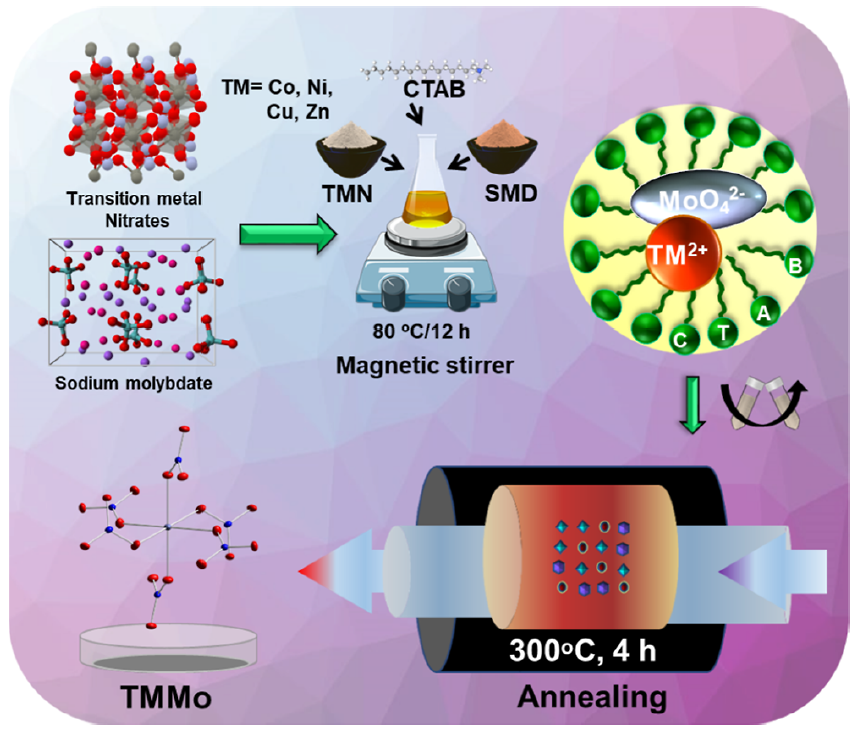
· 논문명 : Role of Physicochemical Features toward Bifunctional Redox Activity of Transition-Metal Molybdates · 저 자 : Jayasmita Jana, Tata Sanjay Kanna Sharma, Jin Suk Chung, Won Mook Choi, Seung Hyun Hur* · 게재지 : ACS Sustainable Chemistry & Engineering (2023, 11, 13013-13023) · 초록 In recent years, the search for non-noble metal-based bi-functional electrode materials with excellent activity and stability for overall water splitting has led the energy field research toward transitionmetal (TM)-based materials that are abundant and comparatively stable over a wide range of pH. Herein, a series of late first-row TM molybdates (TMMo, TM = Co, Ni, Cu, and Zn) were studied for alkaline water splitting, where the materials were synthesized through a surfactant confinement reaction via the hydrothermal process. The microscopic analyses showed varied morphology like, fusiform, forest, disks, and rugby ball with multivalency and monoclinic/ triclinic crystal structures of the TMMo materials. Among the molybdates, CoMo, with a larger number of active sites with an inequivalent atomic position in the cluster and upshifted d/p bands showed the best activity as both cathode and anode materials with the overpotentials of 280 and 408 mV, respectively, to obtain a current density of 100 mA cm−2. CoMo exhibited faster reaction kinetics over other molybdates as it had lower charge-transfer resistance with significant stability. Furthermore, a two-electrode system with CoMo as the cathode and anode provided a lower cell voltage of 1.86 V at 100 mA cm−2 current density over commercial electrodematerials, indicating CoMo can be an excellent commercial alternative electrocatalyst for overall water splitting.
|
|||||
-
이전글
- 이전 게시글이 없습니다.