| (2024.12) The coexistence of highly dispersed Pt2+ and Pt0 co-catalysts on chemically oxidized g–C3N4 via metal-support interaction: The effect of reduction time on Pt species and hydrogen evolution of Pt/g-C3N4 photocatalysts | |||||
| 작성자 | 관리자 | 작성일 | 2025-03-05 | 조회수 | 104 |
|---|---|---|---|---|---|
|
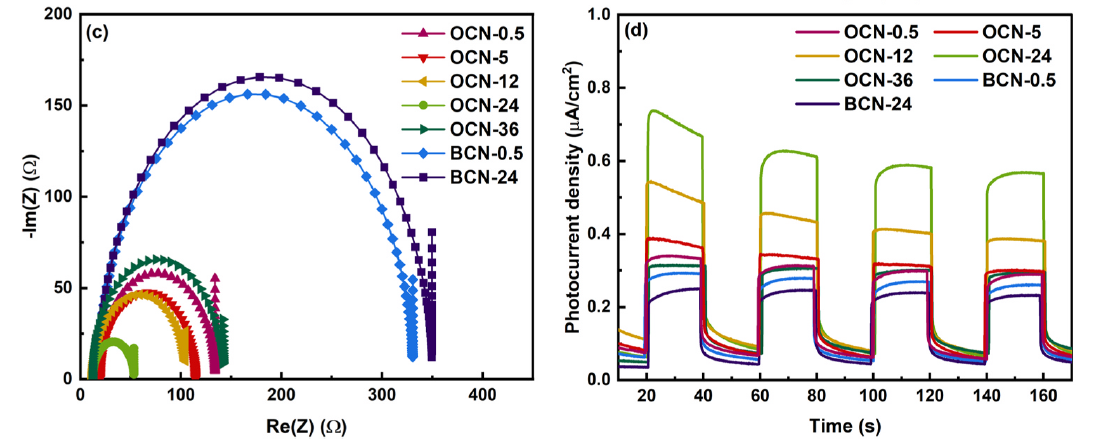
· 논문명 : The coexistence of highly dispersed Pt2+ and Pt0 co-catalysts on chemically oxidized g–C3N4 via metal-support interaction: The effect of reduction time on Pt species and hydrogen evolution of Pt/g-C3N4 photocatalysts · 저 자 : Phuong Anh Nguyen, Thanh Truong Dang, Van Tam Tran, Duc Quang Dao, Thi Van Anh Hoang, Won Mook Choi, Jin Suk Chung*, Eun Woo Shin* · 게재지 : Journal of Alloys and Compounds (2024, 1007, 16392) · 초록 Platinum(Pt) cocatalyst greatly enhances the photoactivity of the graphitic carbon nitride(g-C3N4) photocatalyst for photocatalytic H2 evolution where the Pt properties are critical to determine the photocatalytic activity. In this study, highly dispersed Pt was successfully loaded onto chemically oxidized g-C3N4 (OCN) via a hydrogen reduction process over different reduction time. OCN photocatalysts containing highly dispersed Pt2+/Pt0 exhibited outstanding charge separation efficiency and photocatalytic performance. Symmetrical -C=O groups on the OCN surface specifically interacted with atomic Pt2+, and the Pt2+ atoms were stably reduced to Pt0 atoms along with the generation of -OH groups over time. The coexistence of Pt0 and Pt2+ promoted superior photocatalytic performance because the photoinduced charge transfer was facilitated by the Pt0 atoms, which was evidenced by the DFT calculation and the EIS, PL, and photocurrent response results. Consequently, after the 24 h reduction, the highly stable and active Pt2+/Pt0 atoms were effectively distributed over OCN, resulting in the highest HER activity at 4091.3 μmol g-1h-1.
|
|||||