| (2024.06) Electrochemical Ammonia Oxidation Reaction over Mn/CeO2 (M = Pt, Ir; n = 3, 4) Catalysts | |||||
| 작성자 | 관리자 | 작성일 | 2024-08-14 | 조회수 | 108 |
|---|---|---|---|---|---|
|
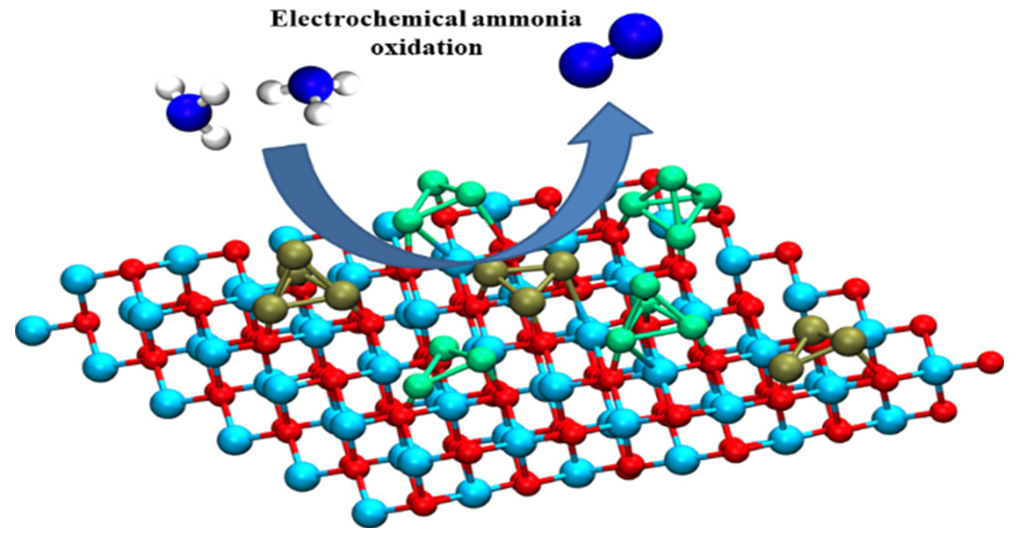
· 논문명 : Electrochemical Ammonia Oxidation Reaction over Mn/CeO2 (M = Pt, Ir; n = 3, 4) Catalysts · 저 자 : K. S. S. V. Prasad Reddy, Jin Suk Chung*, Sung Gu Kang* · 게재지 : Journal of Physical Chemistry C (2024, 128. 25, 10317-10323) · 초록 There exist numerous challenges in hydrogen generation, storage, and transportation. Ammonia has been attractive as an efficient hydrogen carrier by virtue of its high specific density. Designing outstanding catalysts for converting NH3 through the ammonia oxidation reaction (AOR) is significant. Because of the synergistic effects resulting from the interactions between metal and support, it is believed that the support is significant for improving the catalyst’s activity. Owing to this, the activity of small Mn(M = Pt, Ir; n=3, 4) clusters supported by CeO2(111) surfaces was theoreticallyexplored. The Mn/CeO2(111) surfaces kinetically exhibit the G–M reaction mechanism rather than the N + N mechanism. In addition, we determined the range of operating onset potentials over Mn/CeO2(111) surfaces for effective AOR. Using the Sabatier analysis, we also predicted that Pt3/CeO2(111) is the most active catalyst for AOR.
|
|||||