| (2023.11) Synergistic Effects of Ni and Cu in Morphology-Controlled NiCu Electrocatalysts for Ammonia Electro-oxidation | |||||
| 작성자 | 관리자 | 작성일 | 2024-06-10 | 조회수 | 41 |
|---|---|---|---|---|---|
|
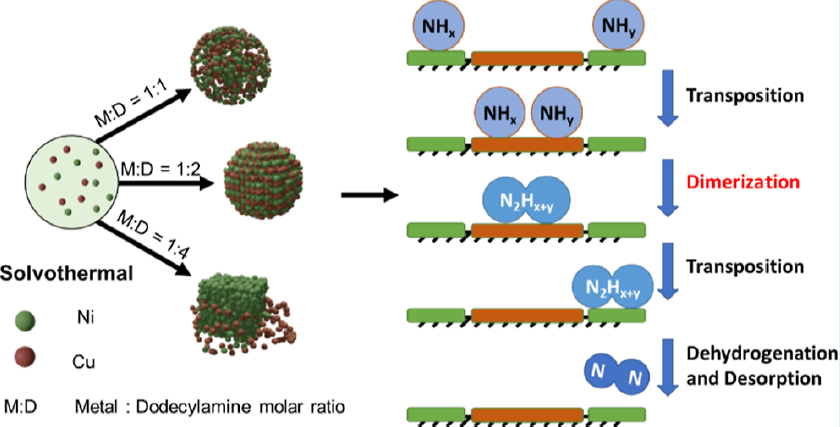
· 논문명 : Synergistic Effects of Ni and Cu in Morphology-Controlled NiCu Electrocatalysts for Ammonia Electro-oxidation · 저 자 : Hoang Khoi Vu, Tahereh Mahvelati-Shamsabadi, Thanh Truong Dang, Seung Hyun Hur,Sung Gu Kang, Jin Suk Chung* · 게재지 : ACS Applied Nano Materials (2023, 6, 20688-20699) · 초록 The ammonia electrochemical oxidation reaction (AOR) has recently attracted attention not only for environmental remediation but a lso for anode reactions in direct ammonia fuel cells and hydrogen fuel production. A deep understanding of the AOR mechanism is of great importance in the design of powerful catalysts. Here, we introduce morphology-controlled electrocatalysts using a facile solvothermal method and dodecylamine as a shape-controlling precipitation agent. Several shapes, including scattering nanoparticles, microspheres, and microcubes, were synthesized by adjusting the ratio of metal precursors to the dodecylamine content. The optimized catalyst, NiCu-D-1:2 with a microsphere shape, showed a hierarchical nanostructure, which provided better contact of the catalyst surface with the reactants and facilitated mass transfer through the reaction. Cyclic voltammetry experiments found that NiCu- D-1:2/CP supplies a current density of 44.9 mA/cm2 at the potential of 0.6 V vs Hg/HgO. Furthermore, NiCu-D-1:2/CP promoted a high Faradaic efficiency of 79% toward N2, which was confirmed using in operando gas chromatography. In addition, a mechanism explaining the synergistic effect of nickel and copper in providing robust AOR activity with a high N2 selectivity is proposed.
|
|||||